All My Artworks Are
Inspired by Nature
Vestibulum mollis nisi at mauris accumsan dapibus. In dictum dui quis sem ullamcorper, eu convallis massa ultricies. Nulla viverra rutrum dolor, at varius lectus ultrices id. Mauris ipsum eros, pulvinar eu elit luctus, cursus mattis libero.


Abstract Acrylic
Duconvallis massa ultricies. Nulla viverra rutrum dolor, at varius lectus ultrices id. Mauris ipsum eros, pulvinar eu elit luctus.
Recent Artworks
Vestibulum mollis nisi at mauris accumsan dapibus. In dictum dui quis sem ullamcorper, eu convallis massa ultricies. Nulla viverra rutrum dolor, at varius lectus ultrices id. Mauris ipsum eros, pulvinar eu elit luctus, cursus mattis libero.
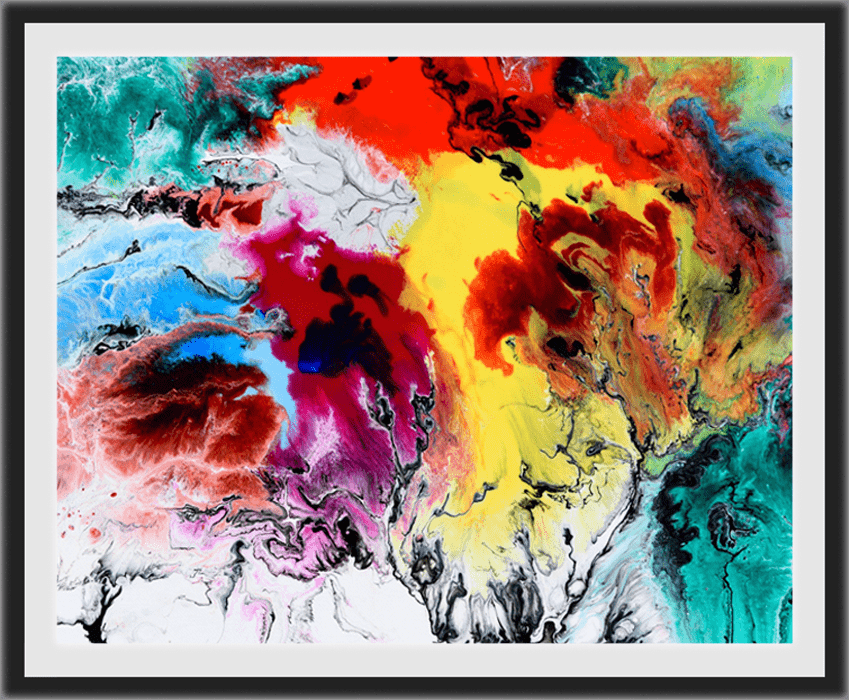
Abstract Artwork
Duconvallis massa ultricies. Nulla viverra rutrum dolor, at varius lectus ultrices id. Mauris ipsum eros, pulvinar eu elit luctus.
My Story
Imperdiet lacus sit amet scelerisque commodo. Praesent nec lectus ante. Nulla ultrices ligula vitae pellentesque ullamcorper. Quisque scelerisque varius mattis. Integer ac libero congue, fermentum odio vel, posuere est. Morbi neque elit, pulvinar eu velit non, maximus bibendum arcu. Ut sollicitudin ante sed aliquam lobortis. Curabitur gravida, ipsum ut malesuada congue, metus tellus ornare dui, eget interdum arcu nisi id elit.
“Donec non ex ultricies, luctus magna ac, auctor felis. Nam ornare, orci vel dapibus dapibus, orci justo lacinia dui, viverra felis neque at sapien.”
Exhibitions
Vestibulum mollis nisi at mauris accumsan dapibus. In dictum dui quis sem ullamcorper, eu convallis massa ultricies. Nulla viverra rutrum dolor, at varius lectus ultrices id. Mauris ipsum eros, pulvinar eu elit luctus, cursus mattis libero. Nulla eget felis elementum, semper purus eu, imperdiet leo.

10 Jun 2018 - 20 Jun 2018
Italian Artist Mega Meet

02 May 2018 - 10 May 2018
Vegas Art Exhibition

12 Apr 2018 - 20 Apr 2018
Lyon Mega Exhibition

20 Feb 2018 - 01 Mar 2018